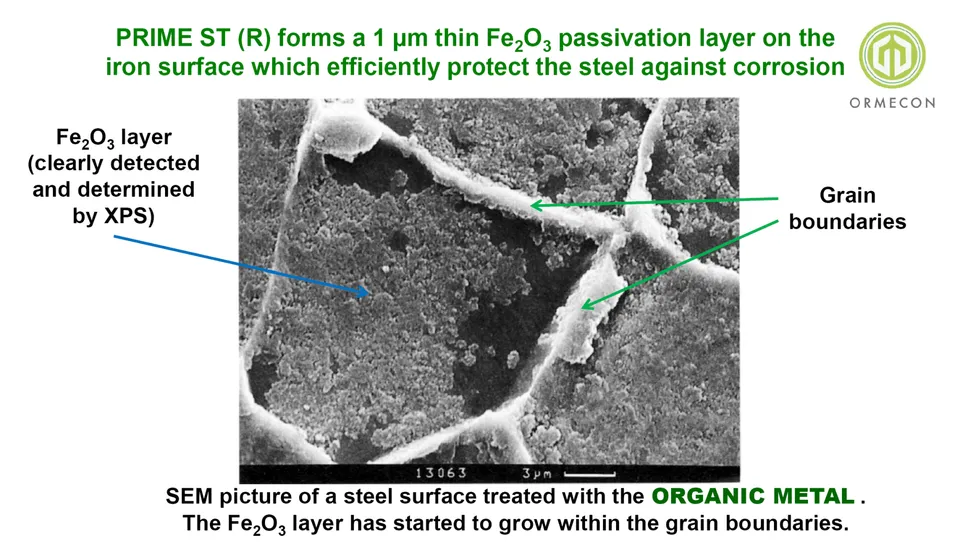
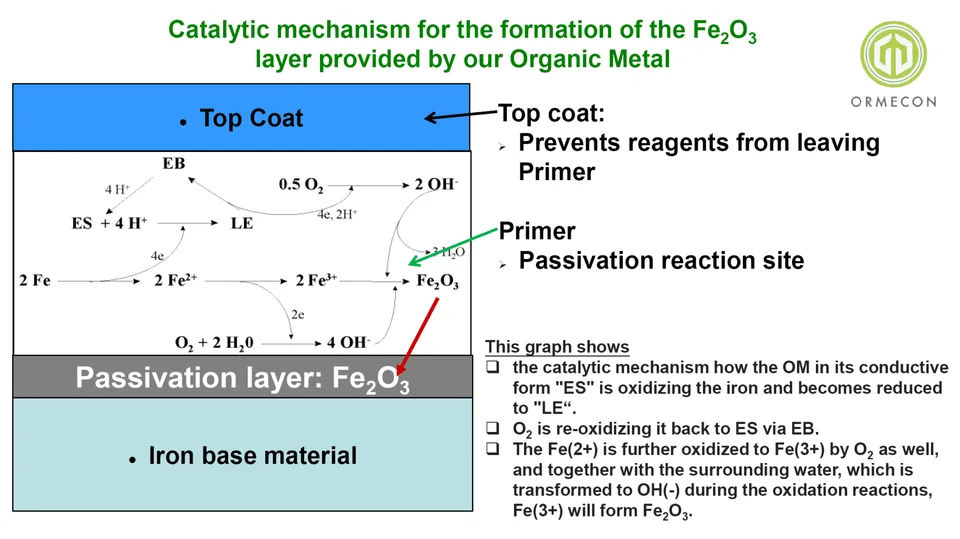
what does "passivation of iron" mean?
how is it possible, what does it do?
Aluminum becomes automatically passivated as soon as the fresh virgin metal is subjected to our normal ambiet atmosphere: it forms a dense and well adhering Al2O3 layer which protects aluminum from becoming corroded: no aluminum „rust“ is formed. Iron (Fe) however does not become passivated, and in fact, there is no process know how one could do that (except for stainless steel under very special conditions).
– Except for now with our Organic Metal, Polyaniline. This new material is both, a metal (in fact: a nanometal) and a catalyst, i.e. that’s very unique because there is no other (conventional) metal which is also a catalyst. Metal ions can be part of catalysts, but a metal itself would not be catalytically active. Our Organic Metal does not loose its form, does not chemically degrade when being reduced and re-oxidized. And that’s what’s happening:
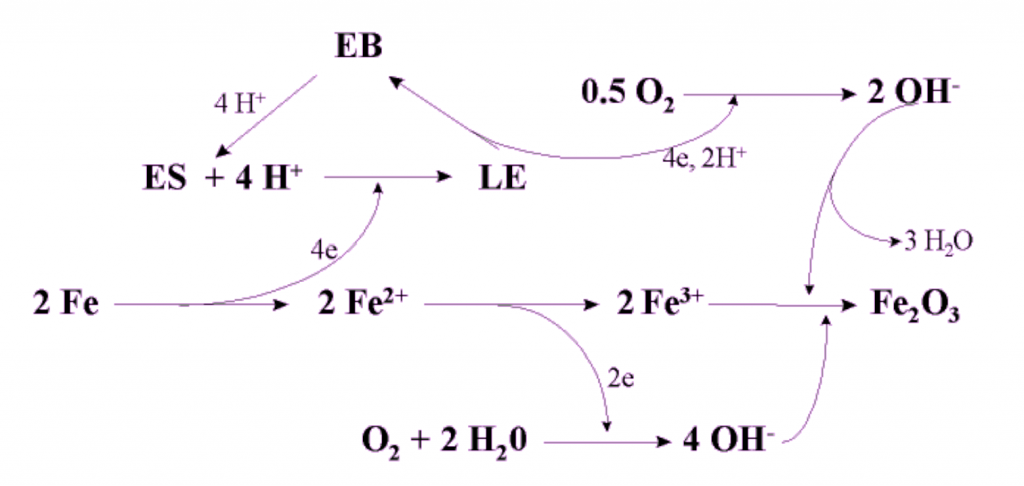
- The Organic Metal (here its chemical name „Emeraldine salt, ES“) oxidized the iron (Fe) to Fe(2+) while itself being reduced to „Leucoemeraldine, LE“)
- Fe(2+) becomes further oxidized to Fe(3+) by oxygen which is also present in paint layers, and oxygen also re-oxidizes the LE to ES (the Organic Metal to begin with) via a neutral form, called Emeraldine base (EB)
- Fe(3+) becomes converted to Fe2O3 by OH(-) which has been formed during the various oxidations steps.
Lool at the below reaction mechanism scheme!
Further information (like: how does the Fe2O3 layer look like?, how should the coating layer be made? etc) can be found in this presentation.
here already quickly 2 key slides: